Refrigerator:
Food preservation was a serious challenge before the discovery of refrigerators. The best options were to suspend spoilable items in the rivers, wells, or at the bottom of ponds. In spite of that, sometimes stable foods were heavily salted, spiced, pickled, canned or dried to prevent bacterial growth.
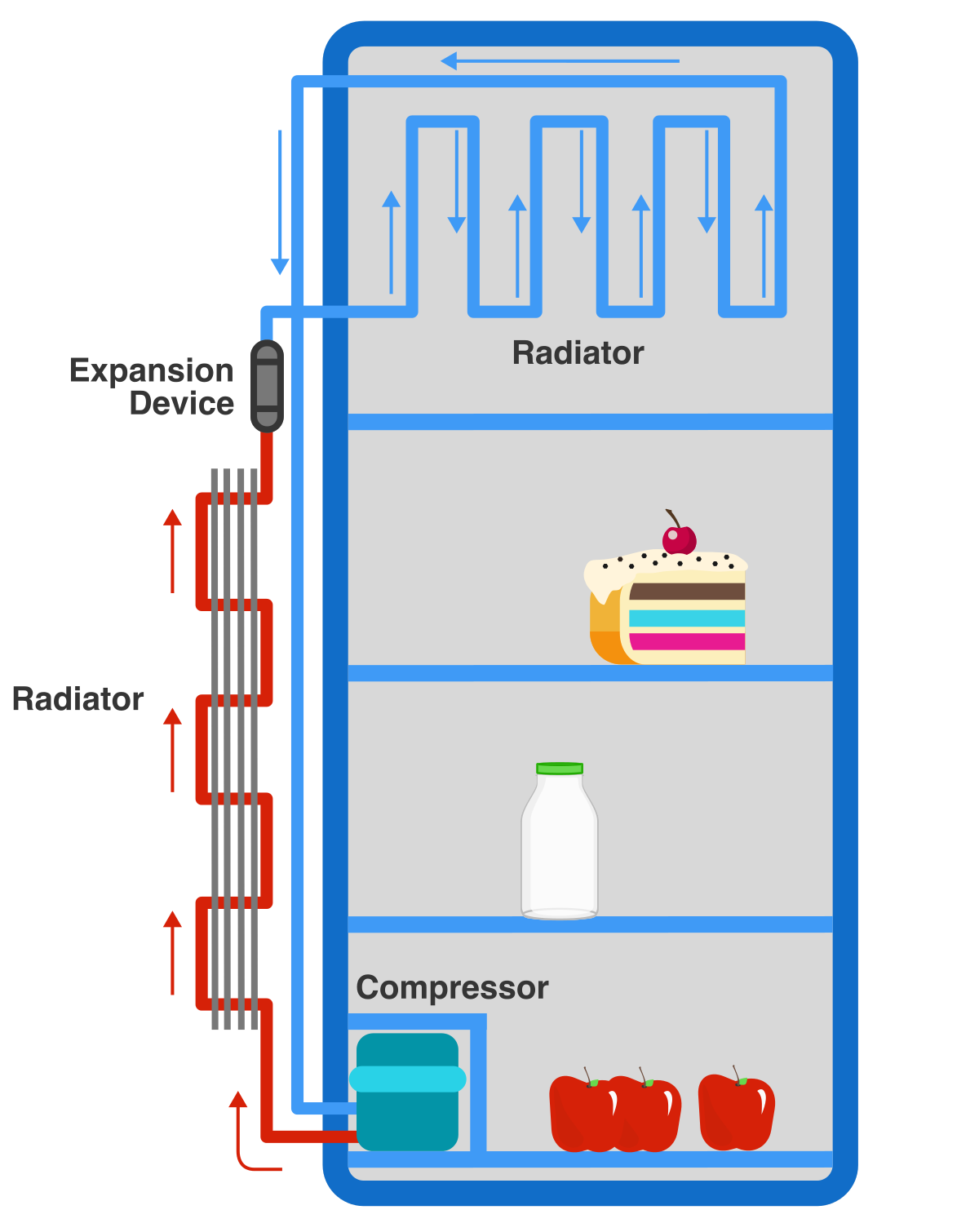
With the industrial age, we began to understand thermodynamic cycles and through an interplay of pumps, valves, and heat exchange; we learned to cool food to arbitrary temperatures using the refrigerator cycle.
So, Let's learn about its three crucial components and understand how they come together to produce the refrigerator’s cooling cycle.
Question: What is the basic idea behind a refrigerator?
Answer: It removes heat from the compartment and pumps it to the outside.Refrigerators continually take away heat from the air in the food compartment and dump it into the environment.
The internal compartment of a refrigerator is approximately closed to the outside and does not exchange gas with it. It is also well insulated, which keeps heat oozing to a minimum.
 While it is possible to make cold air from hot, it requires a lot of energy to do so in any great amount which is referred as WORK!
While it is possible to make cold air from hot, it requires a lot of energy to do so in any great amount which is referred as WORK!As the refrigerator carries out a cycle to move heat out from the food compartment and into the room.
This occurs via three main components.
- a pump that compresses gas
- a valve that expands gas
- radiators that allow gas to exchange heat with the surroundings.
Let's now understand the behavior of each of these components by analogy with everyday phenomena, starting with the compressor pump.
Question: Just after you finish, how does the temperature of the air in the basketball compare to that of the outside?Will it be colder, same temperature or warmer than before?
Answer: It'll be warmer.
Consider what happens when you try to stuff extra clothing into a piece of luggage. Each additional piece requires more work than the last, as the pressure builds up. The same thing is happening with the gas entering the basketball.
First, note what temperature is, it's the average kinetic energy of molecules. Thus, when we add energy to a gas, we're raising the kinetic energy of the molecules.
At atmospheric pressure, gas has a preferred density, but to pump up the ball, we need to keep putting air in until it's well over atmospheric pressure. Gasses resist being compressed like this!!!! And this is why it requires work to force new air molecules into the ball and also the reason that it gets harder to operate the pump as the pressure increases. Thus, when we pump up the ball we're adding energy to the gas, raising its temperature.
As shown in the diagram below, the gas within the basketball has greater speed than the gas outside.

 An analogy for what happens to gas at the expansion valve is what happens to air in an expanding piston. Suppose you have a piston filled with dense gas and the piston is free to expand into a bed of soft clay.
An analogy for what happens to gas at the expansion valve is what happens to air in an expanding piston. Suppose you have a piston filled with dense gas and the piston is free to expand into a bed of soft clay.Question: How does the temperature of the gas in the piston before the expansion compare to the temperature after?
Answer: It will be colder afterwards!
This is essentially the reverse of pumping up the basketball. Since the piston can move, it is possible for the gas to expand by pressing the piston arm into the clay bed. To compress the clay requires the gas to overcome the clay's tendency to maintain its shape and resist deformation. In other words, the gas must do work on the clay, through the piston.
Thus, in expanding its volume, the net energy of the gas is decreased, lowering its temperature.
Finally, we consider a hot balloon in a cold room, an analogy for what happens to gas in a radiator. Suppose you fill a balloon with air that is 40℃ and place it in a room that's 30℃.
Question: Approximately what temperature will the air in the balloon have after a long time?Answer: 30℃
When two objects are left in contact for a long time, they tend to exchange heat until they arrive at the same temperature. In the case of a balloon and a room full of air, the room contains much more heat so the balloon is unable to heat it to any significant degree. Therefore, the balloon will dump heat into the room until they're both very slightly above the starting temperature of the room.
It may seem as though the balloon can't exchange heat with the environment, since it's (relatively) impermeable to gas. However, heat can move across the balloon, and gas molecules on either side can contribute heat to the balloon, which is then picked up by molecules on the other side.
will be continued......



Comments
Post a Comment